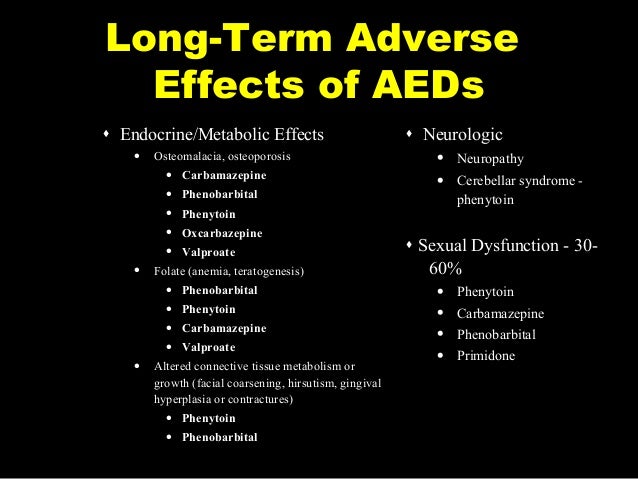
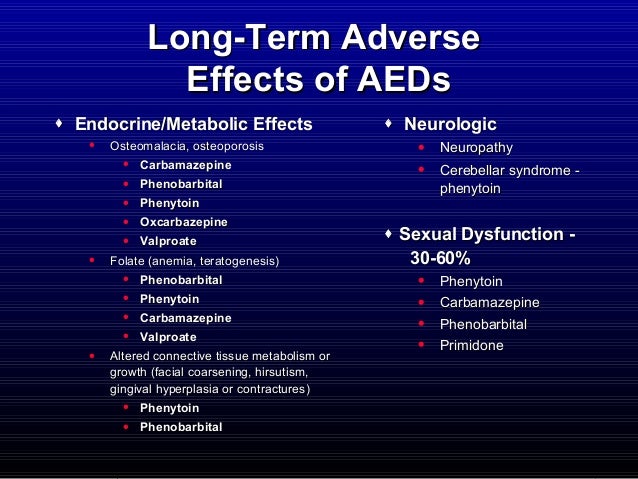
long term side effects of trileptal
 Trileptal Side Effects: Common, Severe, Long Term - Drugs.com
Trileptal Side Effects: Common, Severe, Long Term - Drugs.comCopyright © 2018 by RxList Inc. RxList does not provide medical advice, diagnosis or treatment. .Trileptal Trileptal (Oxcarbazepine) drug center side effects Find lower prices in Oxtellar XRMedical Editor: Trileptal (oxcarbazepine) is a medication, or antiepileptic, used to treat partial seizures in adults and children who are at least 2 years old. Trileptal is available in form. Common side effects of Trileptal include: with Trileptal starts at a dose of 600 mg/day, twice a day. If necessary, the dose can be increased at a maximum of 600 mg/day approximately at weekly intervals; the recommended daily dose is 1200 mg/day. Trileptal can interact with carbamazepine, phenobarbital, phenitoin or . Many other medications can interact with oxcarbazepine. Tell your doctor all the medications and supplements you use. During pregnancy, trileptal should be used only when prescribed. It can damage a fetus. Since untreated seizures are a serious condition that can damage both a pregnant woman and a fetus, do not stop taking this medication unless directed by your doctor. Hormonal control of birth may not work if taken with this medication. Discuss birth control with your doctor. This medicine passes to breast milk but it is unlikely to damage a nursing baby. Talk to your doctor before breastfeeding. Our Trileptal (oxcarbazepine) Side Effects Drug Center provides a complete overview of the available information about possible side effects when taking this medication. This is not a complete list of side effects and others may occur. Call your doctor to advise you about side effects. You may report side effects to FDA at 1-800-FDA-1088. IMAGES Get emergency medical help if you have signs of an allergic reaction: hive, shortness of breath, swelling of the face, lips, tongue, or throat. Signs of an allergic reaction: Look for medical treatment if you have a serious drug reaction that can affect many parts of your body. Symptoms may include: skin rash, fever, swollen glands, flu-like symptoms, muscle aches, severe weakness, unusual or yellowish bruises of the skin or eyes. Look for medical treatment if you have a serious drug reaction that can affect many parts of your body. Oxcarbazepine can reduce sodium in your body to dangerously low levels, which can cause a life-threatening electrolyte imbalance. Call your doctor immediately if you have nausea, lack of energy, confusion, feeling tired or irritable, severe weakness, muscle pain or increased seizures. Call your doctor immediately if you havetell your doctor about any new or worsening symptoms, such as: mood or behavior changes, depression, anxiety, or if you feel agitated, hostile, restless, hyperactive (mentally or physically), or if you have thoughts about suicide or get hurt. Report any new or worsening symptoms to your doctor, such as common side effects may include: This is not a complete list of side effects and others may occur. Call your doctor to advise you about side effects. You may report side effects to FDA at 1-800-FDA-1088. Read all the patient's detailed monograph for EFECTOS SLIDESHOWSIDE The following serious adverse reactions are described below and elsewhere in labelling: WARNING AND ADMINISTRATIONS And AND ADMINISTRATIVES ADMINISTRATION AND WARNING AND ADMINISTRATION AND ADMINISTRATION Clinical Trial Experience Because clinical trials are conducted under variable conditions, adverse reaction rates observed in clinical trials of a the drug cannot be compared directly to the rates in the clinical trials of another drug dependence and cannot reflect the rates observed in practice. Common Adverse Reactions in All Clinical Studies The most common (≥10% more than placebo for adjunctive or low dose for monotherapy) adverse reactions with TRILEPTAL: diplopia, fatigue, nausea, vomiting, ataxia, abnormal vision, headache, nistagmus tremor, and abnormal gait. Approximately 23% of these 1,537 adult patients treatment interrupted due to an adverse reaction. Adverse reactions most commonly associated with discontinued were: dizziness (6.4%), diplopia (5.9%), ataxia (5.2%), vomiting (5.1%), nausea (4.9%), drowsiness (3.8%), headache (2.9%), fatigue (2.1%), abnormal vision (2.1%), tremor (1.8%) (1.7%), rash (1.4%), hyponatremia (1.0%). The most common adverse reactions (≥5%) with TRILEPTAL in these patients were similar to previously treated Patients. Approximately 9% of these 295 adult patients were suspended treatment due to an adverse reaction. The most commonly associated adverse reactions discontinued: dizziness (1.7%), nausea (1.7%), rash (1.7%), headache (1.4%). The most common adverse reactions (≥5%) with TRILEPTAL in these patients were similar to those seen in adults. Approximately 11% of these 456 pediatric patients treatment interrupted due to an adverse reaction. Adverse reactions most commonly associated with discontinued were: drowsiness (2.4%), vomiting (2.0%), ataxia (1.8%), diplopia (1.3%), dizziness (1.3%), fatigue (1.1%), nystagmus (1.1%). The most common adverse reactions (≥5%) with TRILEPTAL in these patients were similar to those of adults. Approximately 9.2 per cent of 152 decontinuous pediatric patients treatment due to an adverse reaction. The most common adverse reactions associated (≥1%) with discontinued were eruptions (5.3%) and maculopapular eruption (1.3%). The most common adverse reactions (≥5%) with TRILEPTAL in these patients were similar to those observed in older children and adults except the infections and infestations that were most frequently seen in these Smaller kids. Approximately 11% of these 241 pediatric patients treatment interrupted due to an adverse reaction. Adverse reactions most commonly associated with discontinued were seizures (3.7%), state epileptic (1.2%), and ataxia (1.2%). Controlled clinical trials of adjunctives Therapy/Monotherapy In adults previously treated with other AEDsTable 3 list adverse reactions that occurred at least 2% of adult patients with epilepsy, treated with TRILEPTAL or placebo as adjunctive treatment and were numerically more common in treated patients with any dose of TRILEPTAL. Table 4 lists adverse reactions in converted patients other AEDs at or at high dosesTRILEPTAL (2400 mg/day) or low doses (300 mg/day) TRILEPTAL. Note that in some of these studies of monotherapy patients disembarked during a phase of preliminary tolerance is not included in the tables. Table 3: Adverse reactions in a controlled clinic Study of Adjunctive Therapy with TRILEPTAL in Adults Table 3: Adverse reactions in a controlled clinic Study of Adjunctive Therapy with TRILEPTAL in Adults Body system/adverse reaction TRILEPTAL Age of two (mg/day) TRILEPTAL 600 N=163 % TRILEPTAL 1200 N=171 % TRILEPTAL 2400 N=126 % Placebo N=166% Body like a whole Fatigue 15 12 15 7 Asthenia 6 3 6 5 Legion Edema 2 1 2 1 Higher weight 1 2 2 1 Feeling abnormal 0 1 2 0 Cardiovascular system Hypotension 0 1 2 0 Digestive system Nausea 15 25 29 10 Vomiting 13 25 36 5 Abdominal pain 10 13 11 5 Diarrhea 5 6 7 6 Dyspepsia 5 5 6 2 Constipation 2 2 6 4 Gastritis 2 1 2 1 Metabolic and nutritional disorders Hyponatremia 3 1 2 1 Musculoskeletal system Muscle weakness 1 2 2 0 Sprains and Strains 0 2 2 1 Nervous System Headache 32 28 26 23 Seas 26 32 49 13 Somnolence 20 28 36 12 Ataxia 9 17 31 5 Nystagmus 7 20 26 5 Abnormal gait 5 10 17 1 Insomnia 4 2 3 1 Tremor 3 8 16 5 Nervous 2 4 2 1 Agitation 1 1 2 1 Abnormal coordination 1 3 2 1 Abnormal 0 0 2 0 Speech disorder 1 1 3 0 Confusion 1 1 2 1 NOS head injuries 1 0 2 1 Dysmetria 1 2 3 0 Abnormal thoughts 0 2 4 0 Respiratory system Rinitis 2 4 5 4 Skiing and appendices Acne 1 2 2 0 Special Sensible Diplopia 14 30 40 5 Vertigo 6 12 15 2 Abnormal vision 6 14 13 4 Abnormal accommodation 0 0 2 0 Body like Comprehensive Cardiovascular SystemMetabolic System and Nutritional DisordersMusculoskeletal SystemNervous SystemRespiratory SystemSkin and Appendages Special SensationsTable 4: Adverse reactions in controlled clinics Monotherapy studies with TRILEPTAL in adults previously treated with other AEDs Table 4: Adverse reactions in controlled clinics Monotherapy studies with TRILEPTAL in adults previously treated with other AEDs Body system/adverse reaction TRILEPTAL 2400 mg/day N=86 % TRILEPTAL 300 mg/day N=86 % Body like a whole Fatigue 21 5 Fever 3 0 Allergy 2 0 Generalized edema 2 1 Breast pain 2 0 Digestive system Nausea 22 7 Vomiting 15 5 Diarrhea 7 5 Dyspepsia 6 1 Anorexia 5 3 Abdominal pain 5 3 Dry Mouth 3 0 Rectum hemorrhagia 2 0 Toothache 2 1 Hemic and lymphatic system Lymphade no pathy 2 0 Infections and Infestations Viral infection 7 5 Infection 2 0 Metabolic and nutritional disorders Hyponatremia 5 0 Thief 2 0 Nervous System Headache 31 15 Seas 28 8 Somnolence 19 5 Anxiety 7 5 Ataxia 7 1 Confusion 7 0 Nervous 7 0 Insomnia 6 3 Tremor 6 3 Amnesia 5 1 Aggravated seizures 5 2 Emotional capacity 3 2 Hypothesis 3 1 Abnormal coordination 2 1 Nystagmus 2 0 Speech disorder 2 0 Respiratory system Upper respiratory infection 10 5 Tosca 5 0 Bronchitis 3 0 Pharyngitis 3 0 Skiing and appendices Hot Flushes 2 1 Purpura 2 0 Special Sensible Abnormal vision 14 2 Diplopia 12 1 Perversion of flavor 5 0 Vertigo 3 0 Earache 2 1 Ear infection NOS 2 0 urogenital and reproductive system urinary tract infection 5 1 Frequency of Mictury 2 1 Vaginitis 2 0 Body as a complete systemDigestiveHemic and lymphatic systemInfections and InfestationsMetabolic and Nutritional DisordersNert system Respiratory systemSystems of skin and appendicesSpecial sensorsImmediacy and reproductive systemClinical study of monotherapy in non-Adult adults Previously treated with other AEDsTable 5 lists adverse reactions in a controlled clinical study of monotherapy in adults not previously treated with other AEDs that occurred in at least 2% of adult patients with epilepsy treated with TRILEPTAL or placebo and were numerically more common in patients treated with TRILEPTAL. Table 5: Adverse reactions in a controlled clinic Monotherapy study with TRILEPTAL in adults not previously treated with other AEDs Table 5: Adverse reactions in a controlled clinic Monotherapy study with TRILEPTAL in adults not previously treated with other AEDs Body system/adverse reaction TRILEPTAL N=55 % Placebo N=49 % Body like a whole Falling NOS 4 0 Digestive system Nausea 16 12 Diarrhea 7 2 Vomiting 7 6 Constipation 5 0 Dyspepsia 5 4 Musculoskeletal system Paint ass 4 2 Nervous System Seas 22 6 Headache 13 10 Ataxia 5 0 Nervous 5 2 Amnesia 4 2 Abnormal coordination 4 2 Tremor 4 0 Respiratory system Upper respiratory infection 7 0 Epistaxis 4 0 Chest infection 4 0 Sinusitis 4 2 Skiing and appendices Rash 4 2 Special Sensible Abnormal vision 4 0 Body as a complete systemDigestiveMusculoskeletal SystemNervous system Respiratory systemSkin and Appendages Senses Specials Controlled Clinical Adjunction Studies Therapy/Monotherapy In pediatric patients previously treated with other AEDsTable 6 list of adverse reactions that occurred at least 2% of pediatric patients with epilepsy treated with TRILEPTAL or placebo as adjunctive treatment and were numerically more common in treated patients with TRILEPTAL. Table 6 Adverse reactions in controlled clinics Adjunctive Therapy/Monotherapy with TRILEPTAL in pediatric patients Previously treated with other AEDs Table 6 Adverse reactions in controlled clinics Adjunctive Therapy/Monotherapy with TRILEPTAL in pediatric patients Previously treated with other AEDs Body system/adverse reaction TRILEPTAL N=171 % Placebo N=139 % Body like a whole Fatigue 13 9 Allergy 2 0 Asthenia 2 1 Digestive system Vomiting 33 14 Nausea 19 5 Constipation 4 1 Dyspepsia 2 0 Nervous System Headache 31 19 Somnolence 31 13 Seas 28 8 Ataxia 13 4 Nystagmus 9 1 Emotional capacity 8 4 Abnormal gait 8 3 Tremor 6 4 Speech disorder 3 1 Concentration with deficiencies 2 1 Convulsions 2 1 Involuntary muscle contractions 2 1 Respiratory system Rinitis 10 9 Pneumonia 2 1 Skiing and appendices Bruges 4 2 Increase in sweat 3 0 Special Sensible Diplopia 17 1 Abnormal vision 13 1 Vertigo 2 0 Body as a complete systemDigestiveNervous SystemRespiratory SystemSkin and Appendages Special sensationsOther events observed in association with management In the following paragraphs, adverse reactions, not included in the tables or previous texts, which occurred in a total of 565 children and 1,574 adults exposed to TRILEPTAL and are reasonably likely to be related to drug use are presented. Common events in the population, events that reflect chronic diseases and events that can reflect concomitant the disease is omitted particularly if it is minor. They are listed in order decreasing the frequency. Because reports cite events observed on the open label and uncontrolled trials, the role of the TRILEPTAL in its causality cannot be resolutely. Body like a whole: fever, discomfort, chest pain precordial, rigors, weight reduction. Body as a whole: cardiovascular system: bradycardia, cardiac insufficiency, brain bleeding, hypertension, posture hypotension, palpitation, Sincope, tachycardia. Cardiovascular system: Digestive system: increased appetite, blood stool, colitis, duodenal ulcer, dysphagia, enteritis, burp, esophagitis, flatulence, gastric ulcer, gyngival bleeding, gum hyperplasia, hematomesis, straight bleeding, hemorrhoids, hypo, oral drying, biliary pain, right hypochondria pain, retching, sialoadenitis, stomatitis, Ulcerative stomatitis. Digestive system:Hematologic and lymphatic system: thrombocytopenia. Hematologic and Lymphatic System:Laboratory Abnormality: Gamma-GT Increase, hyperglycemia, hypocalcemia, hypoglycemia, hypokalemia, elevated liver enzymes, the serum transaminese increased. Laboratory abnormality: Musculoskeletal system: muscle hypertonia.Musculoskeletal System: Nervous system: aggressive reaction, amnesia, anguish, anxiety, apathy, aura, aggravated seizures, delusion, delirium, level of depressed consciousness, dysphony, dystony, emotional ability, euphoria, extrapyramidal disorder, feeling of drunkenness, hemiplegia, hyperkinesia, hyperreflexia, hypoesthesia, hyporeflexia, hypothonia, hysteria, libido decreased, libido increased, manic reaction, migraine, muscle involuntary contractions, nervousness, neuralgia, eye crisis, panic disorder, paralysis, paronyria, personality disorder, psychosis, ptosis, stupor, tetany. Nervous system: Respiratory system: asthma, dyspnea, epistaxis, laryngismus, pleurisy. Respiratory system:Skin and Apendages: acne, alopecia, angioedema, bruising, dermatitis contact, eczema, facial rash, fetish, follicles, heat rash, hot flutes, photosensive reaction, genital pruritus, psoriasis, purpura, erythematous rash, rash, vitiligo, hive. Skin and Appendages: Special sensations: abnormal accommodation, cataract, conjunctive bleeding, edema eye, hemianopia, midriasis, external otitis, photophobia, scotoma, perversion of flavor, tinnitus, xeroftalmia. Special sensations: Surgical and medical procedures: dental procedure oral, female reproductive procedure, musculoskeletal procedure, procedure skin. Surgical and medical procedures: urogenital and reproductive system: disuria, hematuria, intermenstrual bleeding, leucornea, menorrhagia, mictur frequency, kidney pain, urinary tract pain, polyuria, priapism, kidney stones. urogenital and reproductive system:Other: Systemic erythematous lupus. Other:Laboratory Tests Serum sodium levels have been observed below 125 mmol/L in patients treated with TRILEPTAL [see Warnings and ]. The experience of clinical trials indicates that serum sodium levels return to normal when the TRILEPTAL dose is reduced or suspended, or when the patient was treated conservatively (e.g., fluid restriction). ADMINISTRATIONS AND Clinical Trial Laboratory data suggest that The use TRILEPTAL was associated with decreases in T4, without changes in T3 or TSH. Postmarketing Experience The following adverse reactions have been identified during use after TRILEPTAL approval. Because these reactions are reported voluntarily of an uncertain sized population, it is not always possible reliable estimate of frequency or establish a causal relationship with drug exposure. Body as a whole: multi-organ hypersensitivity disorders characterized by such characteristics as rash, fever, lymphdenopathy, abnormal tests of liver function, eosinophilia and arthralgia [see ATTENTION AND #Body as a Whole: Cardiovascular System: Atrioventricular BlockCardiovascular System: Immune System Disorders: Anaphylaxis [verve] WARNING AND ] Immune System Disorders: ADMINISTRATION And digestive system: pancreatitis and/or lipasa and/or increased amylase digestive system:Hematology and lymphatic systems: aplastic anemia [see Hematological and Lymphatic Warnings and Systems: Metabolism and Nutritional Disorders: Hypothyroidism and inappropriate anti-diauretic hormone secretion syndrome (SIADH)Metabolism and Nutritional Disorders: Skin disorders and subcutaneous tissue: erythema multiform, Stevens-Johnson syndrome, toxic epidermal necrolysis [ver ATTENTION AND ], Apretado de la Pustulosis Exantematosa Generalizada (AGEP) Trastornos de fur y suelo subcuáneo: ADMINISTRATION And Musculoskeletal disorders, connective and bone: There have been reports of decreased bone mineral density, osteoporosis and fractures in patients with long-term therapy with TRILEPTAL. Musculoskeletal disorders, connectives and bone: Injuries, poisoning and procedure complications: fallInjury, poisoning and procedure complications: DisarthriaNervous Disorders of the system: Read all FDA that contains information for PREGUNTASRelated HealthRelated Drugs© Trileptal Patient Information is provided by Cerner Multum, Inc. and Trileptal. IMAGESHealth Solutions From Our SponsorsPill Identifier Tool Quick, Easy, Pill Identity Drug Interaction Tool Drug Control Powerful Drugs Pharmacy Locater Tool Including 24 hours, Trileptal Pharmacies Execution: Execution: Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Drug Comparisoning Copyright © 20 RxList does not provide medical advice, diagnosis or treatment. .
Aed new vs old final
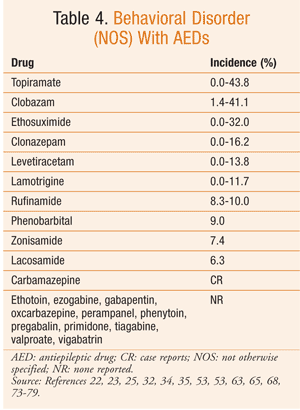
Behavioral Side Effects of Antiepileptic Drugs

Trileptal - NPS MedicineWise

Oxcarbazepine Side Effects: Common, Severe, Long Term - Drugs.com

Trileptal - NPS MedicineWise

Trileptal Full Prescribing Information, Dosage & Side Effects | MIMS Malaysia

Trileptal (Oxcarbazepine): Uses, Dosage, Side Effects, Interactions, Warning

Trileptal (Oxcarbazepine): Uses, Dosage, Side Effects, Interactions, Warning

Trileptal - NPS MedicineWise

Long-term efficacy and safety of adjunctive extended-release oxcarbazepine (Oxtellar XR ® ) in adults with partial-onset seizures – topic of research paper in Clinical medicine. Download scholarly article PDF and read for

Trileptal - NPS MedicineWise
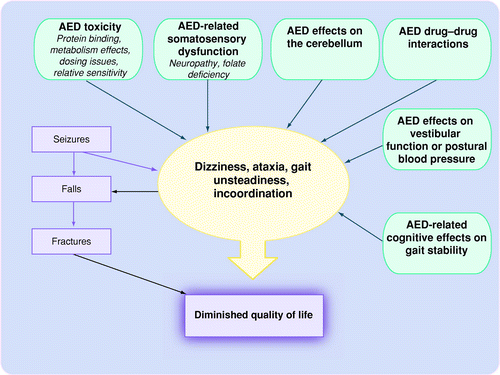
Antiepileptic drugs and their impact on balance | Aging Health

PDF) Effect of Oxcarbazepine on Background EEG Activity and Cognition in Epilepsy

Safety profile of oxcarbazepine: Results from a prescription‐event monitoring study - Buggy - 2010 - Epilepsia - Wiley Online Library
Vol. 8 - Trileptal: A Promising New Mood Stabilizer | The Bipolar Child

Anti-seizure therapy with a long-term, implanted intra-cerebroventricular delivery system for drug-resistant epilepsy: A first-in-man study - EClinicalMedicine

Trileptal - NPS MedicineWise

PDF) Desensitization to Oxcarbazepine: Long-Term Efficacy and Tolerability
Effects of Oxcarbazepine Use on Hemogram, Liver, Thyroid Functions, Lipid Profile in Childhood Epilepsies
Evaluation of hematologic profile may be needed for patients treated with oxcarbazepine
trileptal approved text 1
Anti seizure and rescue medications.updated 8.7.2014
trileptal approved text 1

Sustained Efficacy and Long‐term Safety of Oxcarbazepine: One‐year Open‐label Extension of a Study in Refractory Partial Epilepsy - Beydoun - 2003 - Epilepsia - Wiley Online Library

Trileptal (Oxcarbazepine) for Bipolar Disorder

Case History of Chronic Migraine: Part 2

Behavioral side-effects of levetiracetam in children with epilepsy: A systematic review - ScienceDirect

Trileptal (oxcarbazepine) Drug / Medicine Information

What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs? - Epilepsy & Behavior
/young-woman-mood-collage-629647113-596778e05f9b58161836c498.jpg)
Trileptal Side Effects in Bipolar Disorder
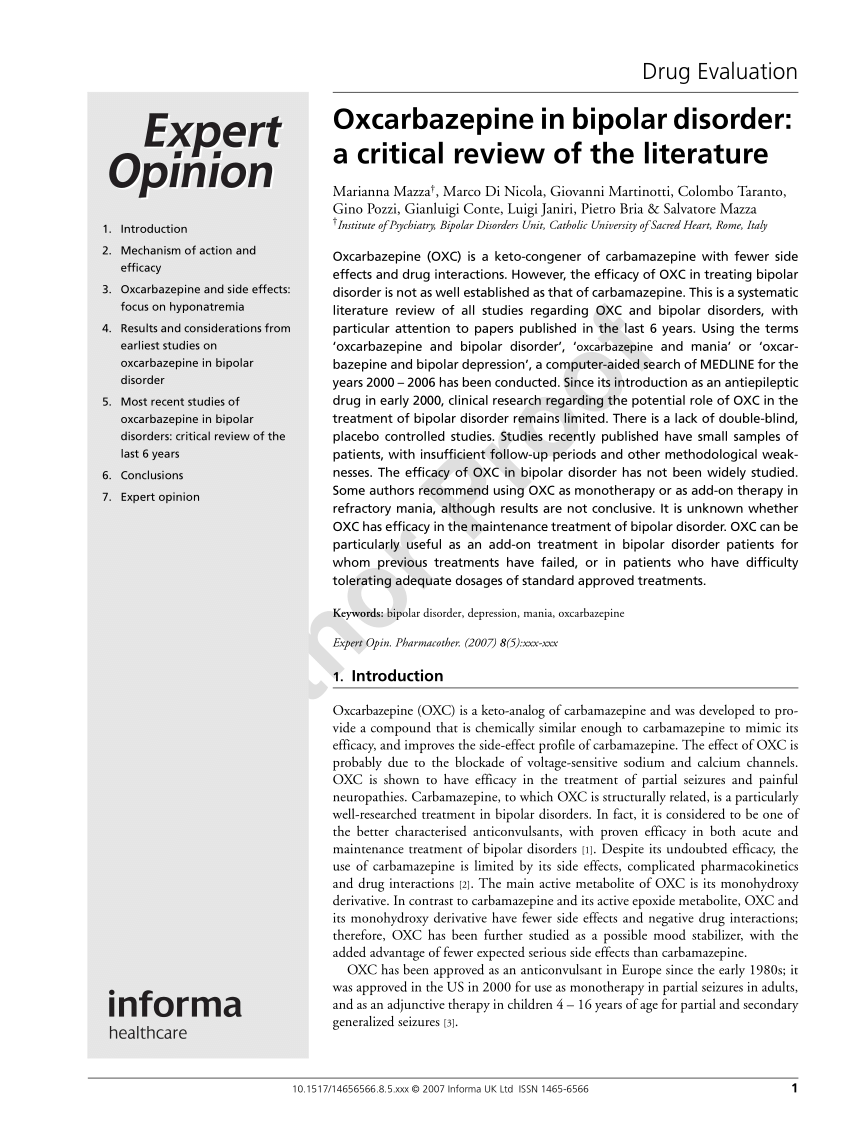
PDF) Oxcarbazepine in bipolar disorder: a critical review of the literature
An Update on the Diagnosis and Treatment of Bipolar Disorder, Part 1: Mania
Oxcarbazepine | SpringerLink

Trileptal Full Prescribing Information, Dosage & Side Effects | MIMS Malaysia

Long-term tolerability, safety and efficacy of adjunctive perampanel in the open-label, dose-ascending Study 231 and extension Study 233 in Japanese patients with epilepsy - Seizure - European Journal of Epilepsy

Pin on Dylan - Leslie
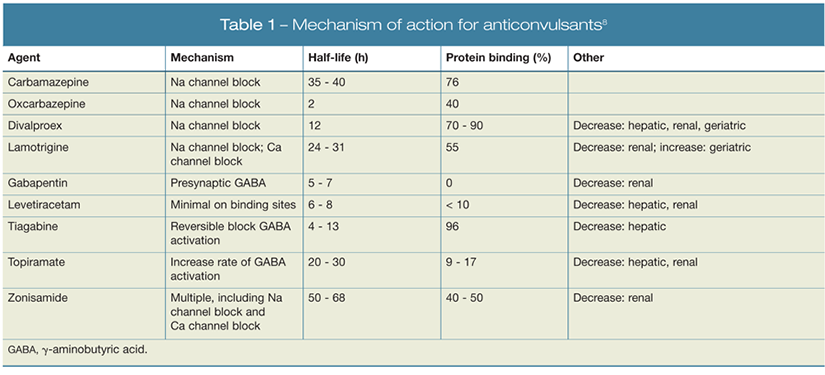
Anticonvulsant Strategies for Treating Bipolar Disorder: What Works, What Doesn't
/lamictal-withdrawal-symptoms-380259_v2-8c92ee1d1cf7493199fad1ba24c0da45.png)
Lamictal Withdrawal Symptoms in Bipolar Disorder

Oxcarbazepine - an overview | ScienceDirect Topics

Antiseizure Drugs (Chapter 21) - Understanding Epilepsy
Posting Komentar untuk "long term side effects of trileptal"